In the field of biology and health technology, sensors that are able to perform time resolved measurements of metabolite concentrations in biological fluids are in great demand. The number of metabolites in humans’ amount to about 2500, and since metabolites are involved in almost all cellular processes, the knowledge of their concentration profiles in time and space can be used to enhance our understanding of biological processes and in biomedical diagnostics. Intracellular metabolite levels play important roles in many cellular events, e.g. in the endosomal-lysosomal system where the monitoring of pH fluctuations are essential to investigate the cellular functions of these compartments. In cancer cells this is of particular interest as this information can guide the design of targeted drug delivery formulations to match the decrease in pH after internalization. Another example is calcium ion regulation that is important in many cellular processes by acting as a second messenger for activating signalling pathways. Also the initiation of programmed cell death has been shown to involve calcium. Another example is the neurotransmitter glutamate, which has been shown to function improperly in patients with Parkinsons disease. It is thus evident that reliable techniques for measuring organelle-associated ion concentrations and fluxes are critical for the ongoing research in these fields.
Intracellular metabolite levels can be measured by the use of fluorescence ratio imaging microscopy (FRIM). We have intracellular metabolite levels by image analysis acquired on a confocal laser scanning microscope (CLSM) equipped with an incubator box and CO2 supply for optimal growth conditions during live cell imaging. However, available sensors for measuring metabolite levels in living cells are not optimal. Nanoparticle based optical sensor technology for quantification of metabolites in living cells has been developed over the last two decades. However, even though these sensor systems have proven themselves as superior to conventional methods, there are still questions about the use of these sensors that need to be addressed, especially regarding sensor design, calibration and image analysis.
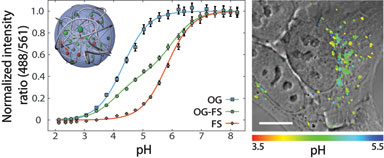
At the DTU Center for Nanomedicine and Theranostics we have developed new nanosensors for measurements of intracellular pH and demonstrated that these are superior to conventional pH sensors. By optimization and improvements of calibration and image analysis we are now demonstrating the use of nanosensors for answering biological questions. Furthermore, we are focussing on expanding these principles to other metabolites. Finally, we are investigating the intracellular trafficking of these nanosensors in order to be able to control the trafficking of nanoparticles inside living cells.