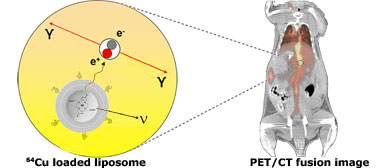
Lipid nanoparticles (liposomes) can serve as diagnostic imaging agents for fast visualization of solid tumors and metastases in vivo due to their potential as carriers of radioactive isotopes for therapeutic use. In this research project radioisotope loaded liposomes are designed for use as imaging agents in clinic for diagnostic and theranostic applications.
As one example, radioactive 64Cu-isotopes (positron emitters) are remote loaded into preformed chelator-containing liposomes. The remote loading approach, concentrates the 64Cu radionuclides in the internal aqueous compartment of the liposomes providing high (>90%) loading efficiencies, where a chelator captures the radionuclides resulting in high radionuclide retention in the liposomes with great in vivo stability. By intravenous (i.v.) administration of 64Cu loaded liposomes into tumor-bearing mice the biodistribution and tumor accumulation have been quantified and visualized directly by positron emission tomography PET/computed tomography (CT) imaging. As the positron-emitter (64Cu) undergoes decay in vivo it emits a positron (e+), which travels a short distance (1-3 mm) before it undergoes annihilation with an electron (e-) producing a pair of 511 keV γ-rays. The γ-rays escape the body and reach the detectors in the PET scanner. Following reconstruction of raw data collected by the PET scanner, the PET image quantitatively reflects the localization and concentration of the 64Cu-liposomes in vivo over time.
One of the great opportunities and advantages of using liposomes as imaging agents is the possibility to design targeted liposomes functionalized with antibodies, peptides or other targeting ligands that recognize over-expressed receptors or antigens on tumor cells or other target cells in vivo. We are investigating a number of imaging applications of targeted and non-targeted radioactive liposomes, where identification of site(s) of disease, grading malignance based on quantifying amount of tracer uptake, measuring the response to different therapeutic modalities, and biodistribution evaluation of liposomal drugs are potential options. The latter gives the opportunity for personalized therapy where treatment benefit is evaluated before administration of liposome drugs. For example, we can administer 64Cu-liposomes and evaluate the ability of therapeutic liposomes to localize at the diseased target site.
Another interesting application of radioactive liposomes investigated in this research project, is the use in internal radiotherapeutic applications, where therapeutic radionuclides (e.g. 177Lu) are loaded into liposomal systems. Here the radioactive liposomes are administered to destroy the tumor cells and cure the patient.
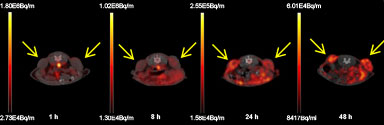
Positron emission tomography (PET)/computed tomography (CT)-images of peptide-targeted
64Cu-liposome distribution in a mouse xenograft model. Axial PET/CT fusion images at 1 h, 8 h, 24 h and 48 h post-injection of
64Cu-liposomes into a mouse with tumors (neuroendocrine carcinoma NCI-H727; marked with arrows) on right and left flank.