– interaction with lipid membranes and its implications in oral drug delivery
Would you rather get an injection or swallow a pill? This Ph.D. project focuses on oral delivery of therapeutic peptides, i.e. small proteins that are routinely used to treat a multitude of diseases.
Oral delivery of peptide drugs rely on three important parameters: stability of the peptides against enzymatic degradation in the intestine, efficient translocation of the peptides across the intestinal cells, and long circulation lifetime in the bloodstream. The first and last criteria can be improved by attaching a fatty acid to the peptide (i.e. acylation), which increases enzymatic stability by blocking restriction sites and increases circulation lifetime by binding to albumin. However, acylation affects the interaction between peptides and cellular membranes - and thereby the peptide translocation - but knowledge about the effect is limited.
A systematic investigation into the effects of acylation will improve the rational design of new acylated peptide drugs. In the project, peptide-membrane interactions are investigated for model lipid membrane and cell membranes, along with the peptide self-association behavior and structure (Fig. 1). The peptide-membrane interaction is correlated to translocation across the intestine through the well-established Caco-2 cell monolayer model, which mimics the intestinal epithelial barrier, and possibly through animal models.
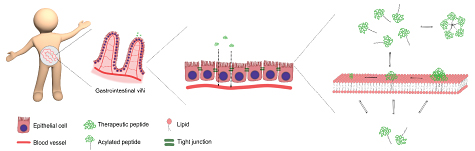
Figure 1 In oral drug delivery of peptide drugs, the peptide is intended to translocate across the intestinal cells and enter the bloodstream. The translocation is greatly affected by interactions between the peptides and the lipid cell membrane, which in turn is influenced by acylation of the peptides.
In the project the acyl chain is varied in a systematic manner while the effects on peptide structure, self-association, membrane insertion and translocation are investigated.