The assembly of carriers containing multiple compartments with a structure remindful of a biological cell is envisioned to be an approach to substitute for missing or lost cellular function, often in the context of enzyme replacement therapy. The aim is to provide a nature-inspired, long term solution for patients suffering from a disease caused by a malfunctioning enzyme.
Carriers Containing Multiple Compartments for Fabry Illness Treatment
The assembly of multicompartment systems with a structure remindful of a biological cell is envisioned to be an efficient tool with potential applications in biomedicine. There is no life without compartmentalization, and living cells developed this strategy to spatially separate different cellular functions within organelles to, for instance, avoid that undesired cross-reactions occur. Carriers containing multiple copies of subcompartments, in which each compartment can be independently loaded with a different compound, are expected to be an optimal form for drug delivery.
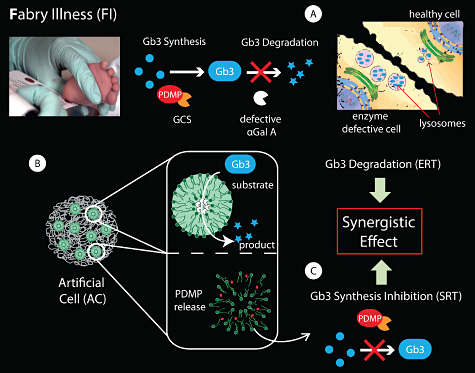
Lysosomal storage diseases (LSD) are a group of ~50 rare disorders which result from defects in lysosomal function, usually as a consequence of deficiency of an enzyme required for the degradation of several metabolites which accumulate in the lysosomes. LSDs, as a group, occur with incidences about 1:5.000 – 1:10.000, affecting mostly children. Fabry-Anderson illness (FI) is a LSD caused by the deficiency of the enzyme α-galactosidase A (αGal A), which results in accumulation of globotriaosylceramide (Gb3) in the lysosomes of endothelial cells, which causes lysosomal expansion and leads to multi-organ pathology (Fig. 1A).
Enzyme replacement therapy (ERT), which involves intravenous injection of the dysfunctional enzyme, remains the only therapeutic option. However, ERT has some important limitations related to toxicity, immune response and fast degradation of the enzyme. Although FI incidence is lower than other illnesses, the high cost of the treatment and the fact that is a treatment for life, makes FI to cause an important impact on the economics of the healthcare system.
The DTU Center for Nanomedicine and Theranostics focuses on developing Artificial Cells (ACs) with the aim to restore cellular and organ functions in FI (Fig. 1B). ACs will be particularly well-suited for the complementary co-encapsulation in separated compartments of enzymes for ERT and small molecules to be used in Substrate Reduction Therapy (SRT). SRT aims to minimize Gb3 storage by inhibiting its synthesis. For FI, the compound PDMP, an inhibitor of glucosylceramide synthase (GCS), has been reported to decrease the biosynthesis of Gb3 (Fig. 1C).
This approach will hold promise to achieve a synergistic effect for the two lines of treatment, allowing for the controlled co-delivery of PDMP without compromising the bioactivity of the encapsulated enzyme αGal A.