Cancer chemotherapy is typically a harsh experience, since the drugs involved are not able to selectively kill cancer cells but will also, to a varying degree, kill normal cells. A joint effort by researchers at Center for Nanomedicine and Theranostics and the German Cancer Research Center (DKFZ) has produced drug leads that hold promise as an effective anti-cancer treatment with minimal or no detrimental effects to healthy cells.
Centrosomes are small cytoplasmic organelles which consist of a pair of centrioles embedded in pericentriolar material and act as microtubule organizing centers. During mitosis, centrosomes function as spindle poles, directing the formation of bipolar spindles, a process essential for accurate chromosome segregation. Centrosomes duplicate precisely once per cell cycle to assure spindle bipolarity, with each daughter cell receiving one centrosome upon cytokinesis. Centrosome amplification is frequent in both solid tumors and hematologic malignancies, and supernumerary centrosomes are linked to tumorigenesis and aneuploidy. The extent of centrosomal aberrations correlates with the degree of chromosomal instability and malignant behaviour in tumor cell lines, mouse tumor models, and human tumors.
Small molecules that selectively inhibit centrosomal clustering, the mechanism that enables cancer cells with supernumerary centrosomes to be viable, hold great promise for cancer chemotherapy. Griseofulvin has been identified as a lead candidate and we have produced more than 100 analogs of the natural product and managed to improve the activity and bioavailability of the compounds. We have also studied the effect of the best leads in both cancer cells and mouse models for colon cancer and multiple myeloma. Furthermore, we have carried out a comparative study of the SAR of selected analogs in a human breast cancer cell line and two pathogenic dermatocytic fungi to shed light on the involvement of tubulin binding in the mode-of-action.
Current research focuses on using chemical probes to identify the target protein of the griseofulvin analogs and further pharmacokinetic investigations aimed at mapping the potential of these compounds as putative selective anticancer drugs.
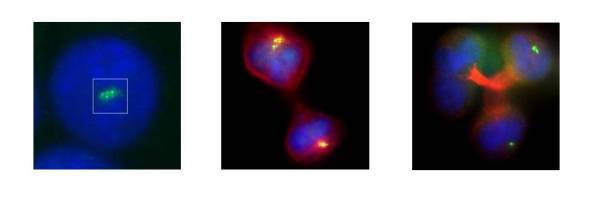
Figure 1 Cancer cell with supernumerary centrosomes (left), undergoing bipolar mitoses through centrosomal clustering (middle) and multipolar mitoses induced by tretment with a grisofulvin analog (right). Staining is α-tubulin (red), centrin (green), and DNA (blue)
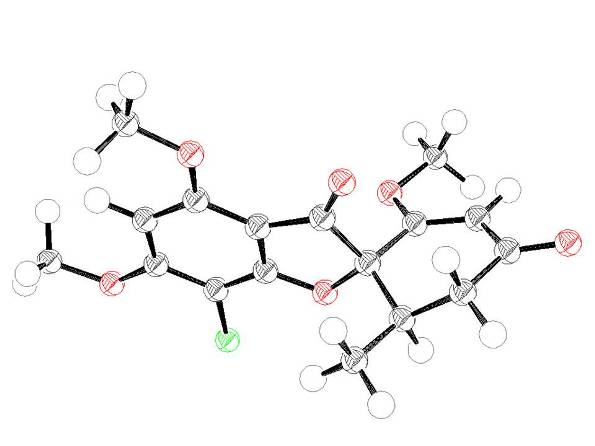 Figure 2 A crystal structure of the original hit compound, the natural product griseofulvin
Figure 2 A crystal structure of the original hit compound, the natural product griseofulvin
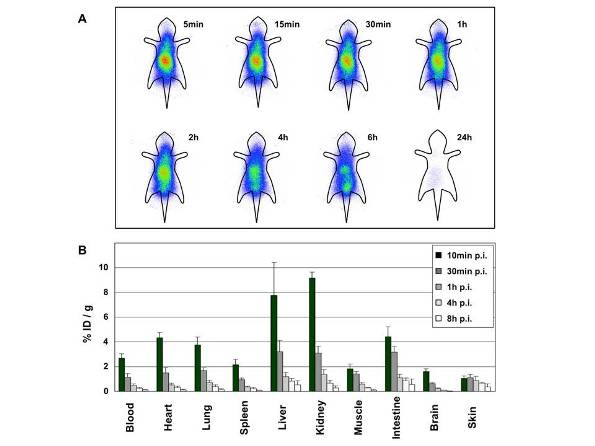 Figure 3 Biodistribution of a griseofulvin analog labelled with radioactive iodine using a new method developed at the Center for Nanomedicine and Theranostics. (A) Whole-body scintigraphic images of NMRI mice at indicated times after intravenous injection of the 125I-labeled griseofulvin analog. (B) Biodistribution of a 131I-labeled griseofulvin analog at different times after intravenous administration to NMRI mice (n = 12). Data are expressed as mean %ID/g +/- SD of each time point
Figure 3 Biodistribution of a griseofulvin analog labelled with radioactive iodine using a new method developed at the Center for Nanomedicine and Theranostics. (A) Whole-body scintigraphic images of NMRI mice at indicated times after intravenous injection of the 125I-labeled griseofulvin analog. (B) Biodistribution of a 131I-labeled griseofulvin analog at different times after intravenous administration to NMRI mice (n = 12). Data are expressed as mean %ID/g +/- SD of each time point